Frequently Asked Questions
- Software & Data Processing
- Scientific
- Manufacturing
- Ordering
- Service
- Field Procedures & Deployment
- Other
Software & Data Processing
The deep SBE41 and SBE61 use the same pressure sensor – a 7000dbar Kistler. And, they are calibrated with the same Paroscientific Digiquartz reference. However, the calibration process is different. A deep SBE41 receives a 2-point sensor only temperature compensation for pressure. The initial accuracy for a deep 41 is +/- 7dbar, typical stability is 2dbar/year. A 61 receives a 4-point temperature compensation for pressure after the instrument is completely assembled, such that the correction includes both the sensor and the electronic boards. The initial accuracy for a 61 is +/-4.5dbar, typical stability is 0.8dbar/year.
Cells that have been contaminated with foreign material generally read low of the actual conductivity. Your zero (in air) conductivity reading is generally unaffected.
The conductivity error due to fouling will generally be proportional to the conductivity value. Conductivity is corrected not as an offset but as a ratio (multiplicative) error compared to a reference.
Salinity is a derivative measurement of temperature, conductivity, and pressure, and should be corrected by adjusting the component measurements. Generally speaking, an error in the conductivity measurement will correlate to a directly proportional error in the salinity measurement.
The temperature and salinity correction for the SUNA can be traced to the experiment outlined in Sakamoto et al. 2009, which is the T/S correction our UCI-based SUNA software uses in post-processing only.
Absorption of UV light in seawater is dominated by dissolved nitrate and bromide ions at wavelengths less than 240 nm. To estimate nitrate, it is necessary to remove the absorption due to bromide. The salinity correction addresses the sea salt extinction coefficients due to bromide. In the real ocean, bromide covaries with NaCl. So, during calibration, we can measure the the bromide absorption due to seawater at one salinity and later predict the absorption due to seawater at any salinity.
Artifical seawater surrogates do not necessarily have the correct bromide absorption to be able to validate the the Sakamoto et al. 2009 salinity correction, so the salinity correction may not product accurate results if your data was not collected in natural seawater.
The measurement range and accuracy of the SBE43 are not definite in discrete units (mL/L, mg/L, µMol/L, etc.) Rather, the range and accuracy specification are expressed as a ratio of the calculated oxygen saturation point. This saturation value depends on the temperature and salinity of the water, decreasing in higher temperatures and higher salinities.
The lower limit of detection of dissolved oxygen concentration is going to be constrained by the accuracy specification, which is a ratio of the calculated oxygen saturation (initial accuracy: +/- 2% of saturation). This saturation value varies depending on the temperature and salinity of the water. Common oxygen saturation values in seawater are in the range of 4-7 mL/L. Using this value as an estimate, you can extrapolate that the accuracy of the sensor in many conditions can be as low as +/- 0.1 mL/L. Therefore, the close you get to zero oxygen concentration, the closer the resolution of your measurement will be to the accuracy spec, until you get below 0.1 – 0.2 mL/L and your measured value is smaller than the nominal error margin.
While the theoretical lower limit of the instrument’s measurement range is zero, the measurement becomes less meaningful the closer your dissolved oxygen measurement gets to 2% of the calculated saturation. For example, if you were in an environment where the oxygen saturation was 7 mL/L but the oxygen concentration measured by the SBE43 was 0.28 mL/L, then your accuracy would effectively be +/- 50% of the measurement.
First off, let’s see what a relatively good Raw Data Plot looks like.
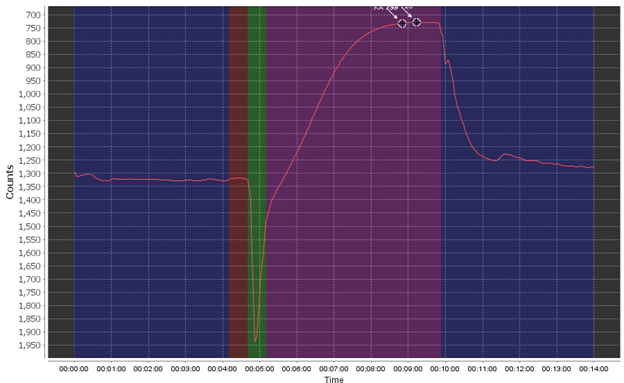
The different phases are the pre-flush phase (dark blue), the ambient baseline reading period (red), the sample reagent pump phase (green), the sample read/reaction phase (purple), and finally the post-flush phase (dark blue). During a “spiked run” the full set of phases are repeated twice.
Using this as an example, you want to see a flat pre- and post-pump phase, a stable (flat) ambient read phase, and a pronounced smooth reaction curve. You may see small bumps here and there due to very small bubbles or other effects but this can be negligible.
The most common failure mode is the bubble spike flag, which triggers when air is introduced into the system, most commonly through the sample line but possibly through incorrectly installed cartridges or tubing.
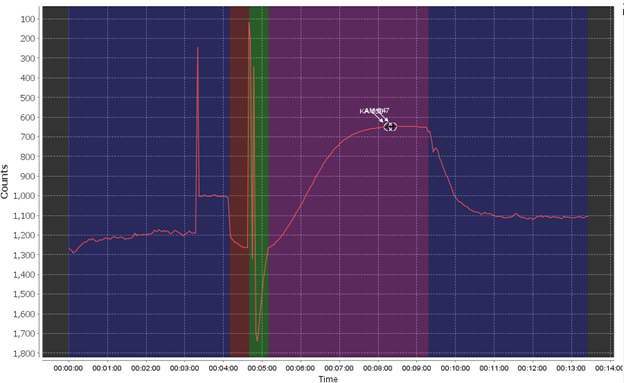
The bubble failure is typically identified by large jumps and spikes randomly in the data, primarily in the pre- and post- pump phase and the ambient read phase. Air bubbles create an unstable base line (red) which will affect the sensor’s accuracy. In extreme examples it can completely overshadow the reaction curve as well or shift the baseline to the point where your reaction creates a negative value. Air bubbles often exacerbate other issues, like weak pumps, clogged filters, or already low phosphate in your sample.
Since bubbles can come from a few different places there are multiple checks you can do to try to stop bubbles from appearing in your data. The most important check is ensuring that your hydrocycle is fully submerged for the entirety of your deployment, as the sample line being exposed to air will introduce bubbles.
You should check that each of your reagent carts are fully installed. Press down on each reagent cart until you can hear them click and be careful about applying too much force to any of the plastic pieces that may crack and allow for leaks or air.
You can also test the pump volumes of each reagent and sample to see if your filters are clogged or if there is another issue causing the sample injection system to fail, like a leak. You do so by using Cyclehost’s Pump controls. You should choose all pumps and run each for 100 pumps. A healthy system will pump 1-1.3 ml of reagent and 2.5-3 ml of sample.
If you have bubbles already and need to recover, the most thorough method is to follow our maintenance and cleaning instructions in the manual, primarily the extended flush. Running the extended flush multiple times should be enough to clear bubbles from your system if no other issues are evident.
If doing so still fails to recover your instrument’s counts please reach out to technical support with a copy of your hydrocycle data and brief timeline of events.
Scientific
The ECO sensors primarily image a volume that is approximately 1 cm3, centered 1 cm off the face of the instrument.
NOTE: This does not preclude return of photons from outside of this volume and in particular it is best that no fixed objects are in the field of view of the instrument, such as cables or cage hardware.
We recommend that the instruments be mounted in such a way that they are seeing only the free field. Often when mounted on a CTD, mounting the ECO face-down or away from the CTD can improve reading accuracy.
That said, the ECO’s have been used in fairly tight quarters with excellent results, including in flow-through housings and dense instrument cages.
When the ECO is mounted in such a way that there is an object in front of the Instrument, the ‘wall effect’ should be established. We are assuming that the material does not fluoresce.
To establish the “wall effect”:
1) Turn the instrument on in air, or better, in a clear water bath, and collect an ‘offset’ reading.
2) Compare this to the factory calibration offset. If the difference is small (e.g. a few counts or mV), then no further action is necessary.
3) Rotate the instrument to find the minimum offset. The backscattering or turbidity channel is the best for this.
4) Mark this position and record the output values of all channels.
5) To minimize the wall effect any object in front of the face of the instrument should be dull black or grey. Tape is usually the easiest solution for this on frames. Grey or black matte plastic is the solution for underway systems.
6) Use these values as the offset values in generating engineering unit output, as specified on the characterization sheets:
Output = ScaleFactor x [InstrumentOutput – Offset]
7) After collecting data from the field, check to confirm that the offset you are using is appropriate. You may find that your minimum values are lower than the offset you have established.
The deep SBE41 and SBE61 use the same pressure sensor – a 7000dbar Kistler. And, they are calibrated with the same Paroscientific Digiquartz reference. However, the calibration process is different. A deep SBE41 receives a 2-point sensor only temperature compensation for pressure. The initial accuracy for a deep 41 is +/- 7dbar, typical stability is 2dbar/year. A 61 receives a 4-point temperature compensation for pressure after the instrument is completely assembled, such that the correction includes both the sensor and the electronic boards. The initial accuracy for a 61 is +/-4.5dbar, typical stability is 0.8dbar/year.
The pH sensor will be shipped dry but was pre-conditioned in seawater (generally from Pacific Ocean waters near Hawaii). While conditioning and evaluating the pH sensor, only expose it filtered, sterilized natural seawater. Do not use seawater CRMs (Certified Reference Material), synthetic seawater, deionized water, NaCl Solutions, or tap water.
Before pre-deployment testing, you will need to fill the plumbing around the pH sensor with natural seawater. The pH sensor needs time to acclimate to the ionic concentration of region specific waters. Once wet, the time to recondition the sensor so that it will report within its accuracy specification depends on several factors, including the ionic composition of the seawater used and the amount of time the pH sensor was stored dry. This time can range from several hours to up to three days.
When the seawater bridge between Counter Electrode and ISFET is broken for longer than 10 seconds, it will be necessary to re-condition the sensor. The sensor does not require recalibration after being re-conditioned.
To prepare the sensor for deployment, it is recommended that several days prior to deployment, the isolated battery is connected via the float interface and the pH sensor is stored in water that is similar to the deployment site. The sensor should be stored dry to avoid bio-fouling of the ISFET and the battery may be removed during storage. Seawater creates a half cell bridge between the Counter Electrode and ISFET, and power to that circuit is provided by the isolated 9V cell. Without seawater, the battery is unnecessary and may be disconnected.
Cells that have been contaminated with foreign material generally read low of the actual conductivity. Your zero (in air) conductivity reading is generally unaffected.
The conductivity error due to fouling will generally be proportional to the conductivity value. Conductivity is corrected not as an offset but as a ratio (multiplicative) error compared to a reference.
Salinity is a derivative measurement of temperature, conductivity, and pressure, and should be corrected by adjusting the component measurements. Generally speaking, an error in the conductivity measurement will correlate to a directly proportional error in the salinity measurement.
The temperature and salinity correction for the SUNA can be traced to the experiment outlined in Sakamoto et al. 2009, which is the T/S correction our UCI-based SUNA software uses in post-processing only.
Absorption of UV light in seawater is dominated by dissolved nitrate and bromide ions at wavelengths less than 240 nm. To estimate nitrate, it is necessary to remove the absorption due to bromide. The salinity correction addresses the sea salt extinction coefficients due to bromide. In the real ocean, bromide covaries with NaCl. So, during calibration, we can measure the the bromide absorption due to seawater at one salinity and later predict the absorption due to seawater at any salinity.
Artifical seawater surrogates do not necessarily have the correct bromide absorption to be able to validate the the Sakamoto et al. 2009 salinity correction, so the salinity correction may not product accurate results if your data was not collected in natural seawater.
The SeaFET and SeapHOx systems are designed to sample at a fixed depth. If you want to run discreet samples at depth intervals, you will need to find a way to move the system to a specific depth before each sample interval and stop the descent / ascent for the entire pumping and sampling cycle to get a valid CTD / pH / Oxygen sample.
If you are able to communicate with the system through the serial I/O during profiling, you can send a sampling command to the sensor at each depth point and allow it to complete its sample cycle. Consult the manual for each model for the length of time required to complete each sample. Once the sensor provides a sample, you can then move it to the next depth point and repeat.
If you aren’t running real-time communications to the SeaFET/SeapHOx, you could also set it to autonomously sample at a time interval that gives you enough time to move the package to a new depth point between sample cycles. The challenges with this approach would be to know exactly when the sensor is sampling without any direct feedback from the instrument.
SUNAs ordered with the 5mm path length coupler as a factory option will perform much better in low light transmission waters due to the shorter length the light needs to travel leading to less absorption. Equipping your SUNA with the factory bio-wiper option will also perform better and be less susceptible biofouling or buildup of other material that can reduce light transmission.
There are also some maintenance practices and device settings that can give SUNA a better probability of being able to capture enough light for a sample. Enable adaptive integration will trigger the SUNA to increase the lamp on time when light received by the spectrometer is low. It is also important to clean the windows as frequently as possible and monitor lens for scratches. Finally, you want your maximum light spectral counts at the peak wavelength (around 240nm) to be between 45,000 and 55,000 counts in pure or deionized water. This can be viewed in the “Spectra” tab in UCI when sampling or replaying data. If your peak spectrometer output is below 45,000 counts after cleaning the window, you may increase the integration period by 25 to 50 ms if needed (but not more; further changes require a factory recalibration). After adjusting the integration period, always perform a reference spectrum update per the instructions in the SUNA manual.
The ISFET has two reference electrodes: an internal reference and an external reference, that give separate reference potentials to the ISFET and show separate pH values (pH Internal and pH External). After the corrections for temperature and salinity are applied, the values from the internal and external are similar, and let the user verify the validity of the sensor’s measurements.
Internal reference:
The internal reference electrode inside the DuraFET® is immersed in a bath of saturated potassium chloride (KCl) gel and is physically separated from the environment. The KCl gel exposes the Ag/AgCl internal electrode to a relatively constant chloride concentration. The sensor can therefore measure pH regardless of environmental salinity. If accurate salinity and temperature data are not available, the internal cell is generally more accurate.
External reference:
The external reference electrode has a Ag/AgCl reference electrode in direct contact with seawater. The potential of this electrode varies with pH and chloride concentration, so unless the chloride concentration is known, the external reference is not stable. To correct this, salinity can act as an approximation of chloride concentration. If accurate salinity data is available, it can be applied to the pH external data and significantly reduce measurement errors, and give the most accurate and stable pH data.
Artificial seawater is problematic because the salts in artificial seawater do not completely dissolve leaving you with a solution that does not completely match the ionic concentration of seawater. This is true for synthetic blends (made for aquariums) or ones made from drying natural seawater. The different ionic concentration leads to drift and potential offsets in the pH sensor. This leads to an inaccurate K0 and initial drift in the external reference of the pH sensor, the internal reference electrode should be unaffected because it separated from the seawater by a saturated solution of KCl gel. This occurs because the pH sensor external reference electrode is a solid state AgCl electrode which is in direct contact with the seawater. A unconditioned Ag/Cl external electrode K0 typically changes by 3mV (60mpH) when exposed to natural seawater for the first time. The challenge here is without knowing the ionic composition of the artificial seawater it is difficult to determine exactly how long the drift will occur or if there will be a permanent offset. This is why we recommend to only use natural seawater.
When the Ag/AgCl is first installed in the sensor it is pure Ag/AgCl, but when it is exposed to seawater it reacts with the ions (Br- mostly, but there are other ions too) in seawater which changes its ionic composition and its standard potential. The standard potential is the K0 coefficient which is provided to the customer and the manufacturer the sensors in seawater for ~3 days in natural seawater to ensure the external electrode is conditioned to seawater prior to K0 calibration in seawater baths. If the sensor is then put into artificial seawater with the incorrect ionic compositions, you risk deconditioning the external reference which could lead to inaccurate measurements and drift in the sensor when first deployed. The sensor should recondition to seawater after deployment. The reconditioning can take 3 days to 2 weeks, however permanent offsets can remain even after the external reference has stabilized.
However, if you cannot obtain natural seawater on a regular basis. You could obtain some natural seawater when the pH sensor is recovered or deployed. The seawater could be taken back to your lab, filtered and stored in a dark container in a cool and dark place for a couple months. Just be sure to filter the seawater again before you fill the wet cap to store the pH sensor for long periods of time.
The measurement range and accuracy of the SBE43 are not defined in discrete units (mL/L, mg/L, µMol/L, etc.) Rather, the range and accuracy specification are expressed as a ratio of the calculated oxygen saturation point. This saturation value depends on the temperature and salinity of the water, decreasing in higher temperatures and higher salinities.
The more detailed answer is a theoretical maximum value can be calculated. While the absolute maximum value would need to be calculated using the highest value one could select as a reference saturation point in natural waters, this theoretical maximum value’s calculation is demonstrated below:
10.84 ml/l (15.49 mg/l) the oxygen saturation point for
In the coldest (-2°C) and least saline (0 PSU) water, the oxygen saturation point is 10.84 ml/l (15.49 mg/l. Using this as a reference saturation point, 10.84 ml/l is multiplied by 1.2 to make 13.01 ml/l (18.59 mg/l), or 120% of the oxygen saturation; this represents the upper end of the measurement range in the given water and can be called the theoretical maximum value for the sensor.
The measurement range and accuracy of the SBE43 are not definite in discrete units (mL/L, mg/L, µMol/L, etc.) Rather, the range and accuracy specification are expressed as a ratio of the calculated oxygen saturation point. This saturation value depends on the temperature and salinity of the water, decreasing in higher temperatures and higher salinities.
The lower limit of detection of dissolved oxygen concentration is going to be constrained by the accuracy specification, which is a ratio of the calculated oxygen saturation (initial accuracy: +/- 2% of saturation). This saturation value varies depending on the temperature and salinity of the water. Common oxygen saturation values in seawater are in the range of 4-7 mL/L. Using this value as an estimate, you can extrapolate that the accuracy of the sensor in many conditions can be as low as +/- 0.1 mL/L. Therefore, the close you get to zero oxygen concentration, the closer the resolution of your measurement will be to the accuracy spec, until you get below 0.1 – 0.2 mL/L and your measured value is smaller than the nominal error margin.
While the theoretical lower limit of the instrument’s measurement range is zero, the measurement becomes less meaningful the closer your dissolved oxygen measurement gets to 2% of the calculated saturation. For example, if you were in an environment where the oxygen saturation was 7 mL/L but the oxygen concentration measured by the SBE43 was 0.28 mL/L, then your accuracy would effectively be +/- 50% of the measurement.
The ECO-PAR, due to the nature of PAR sensors, cannot be accurately calibrated outside of the Sea-Bird facility. However, there are some functionality tests that can aid in pre-deployment.
A bright flashlight can validate whether the instrument sees light at all.
On a bench test one should see between 1000-4000 counts normally with the instrument in the white cap standing up on the benchtop. Use a terminal program to see the raw counts, such as Tera Term, and point a flashlight beam near the white cap. Doing so with a functioning unit will cause the counts to go to approximately a couple of thousand in a hair trigger fashion. It should be possible to decrease the counts on a properly functioning instrument by cupping ones hands around the white cap to shield it from light. It should be easy to get a response of a couple of hundred counts total in doing this. In the field if you shine a flashlight beam directly into the optics will see low level ambient light and it is easy to regulate the output in counts at the low end of the range (less than 1000 counts) when it is functioning normally. If your unit is not properly functioning, it will go from 50-ish to a couple of thousand counts and it will be very difficult or impossible to get an output of a couple of hundred counts. While the ranges of the response may differ between PAR models, these tests can be used with other PAR sensors to verify operation.
The ECO-PAR, due to the nature of PAR sensors, cannot be accurately calibrated outside of the Sea-Bird facility. However, there are some functionality tests that can aid in pre-deployment. A bright flashlight can validate whether the instrument sees light at all.
On a bench test one should see between 1000-4000 counts normally with the instrument in the white cap standing up on the benchtop. Use a terminal program to see the raw counts, such as Tera Term, and point a flashlight beam near the white cap. Doing so with a functioning unit will cause the counts to go to approximately a couple of thousand in a hair trigger fashion. It should be possible to decrease the counts on a properly functioning instrument by cupping ones hands around the white cap to shield it from light. It should be easy to get a response of a couple of hundred counts total in doing this. In the field if you shine a flashlight beam directly into the optics will see low level ambient light and it is easy to regulate the output in counts at the low end of the range (less than 1000 counts) when it is functioning normally. If your unit is not properly functioning, it will go from 50-ish to a couple of thousand counts and it will be very difficult or impossible to get an output of a couple of hundred counts. While the ranges of the response may differ between PAR models, these tests can be used with other PAR sensors to verify operation.
There are two optional modifications that can be done to your 9p at our facility (during service or as part of your original order) that will allow the 9p CTD to operate in freshwater deployments. The 9p’s pump will not operate in freshwater without these options.
First, the Modem Pump Control, allows you to control the pump directly, bypassing the requirement for it to see a certain conductivity frequency to activate. (On other CTD’s we can change this conductivity value to allow both freshwater and saltwater).
The second option is the freshwater contact pin, an optional pin modification that allows for the detection of fresh water by the 9p.
Cells that have been contaminated with foreign material generally read low of the actual conductivity. Your zero (in air) conductivity reading is generally unaffected.
The conductivity error due to fouling will generally be proportional to the conductivity value. Conductivity is corrected not as an offset but as a ratio (multiplicative) error compared to a reference.
Salinity is a derivative measurement of temperature, conductivity, and pressure, and should be corrected by adjusting the component measurements. Generally speaking, an error in the conductivity measurement will correlate to a directly proportional error in the salinity measurement.
The temperature and salinity correction for the SUNA can be traced to the experiment outlined in Sakamoto et al. 2009, which is the T/S correction our UCI-based SUNA software uses in post-processing only.
Absorption of UV light in seawater is dominated by dissolved nitrate and bromide ions at wavelengths less than 240 nm. To estimate nitrate, it is necessary to remove the absorption due to bromide. The salinity correction addresses the sea salt extinction coefficients due to bromide. In the real ocean, bromide covaries with NaCl, so we can measure (during calibration) the the bromide absorption due to seawater at one salinity and later predict the absorption due to seawater at any salinity.
Artifical seawater surrogates do not necessarily have the correct bromide absorption to be able to validate the the Sakamoto et al. 2009 salinity correction, so the salinity correction may not product accurate results if your data was not collected in natural seawater.
The measurement range and accuracy of the SBE43 are not defined in discrete units (mL/L, mg/L, µMol/L, etc.) Rather, the range and accuracy specification are expressed as a ratio of the calculated oxygen saturation point. This saturation value depends on the temperature and salinity of the water, decreasing in higher temperatures and higher salinity.
The lower limit of detection of dissolved oxygen concentration is going to be constrained by the accuracy specification, which is a ratio of the calculated oxygen saturation (initial accuracy: +/- 2% of saturation). This saturation value varies depending on the temperature and salinity of the water. Common oxygen saturation values in seawater are in the range of 4-7 mL/L. Using this value as an estimate, you can extrapolate that the accuracy of the sensor in many conditions can be as low as +/- 0.1 mL/L. Therefore, the close you get to zero oxygen concentration, the closer the resolution of your measurement will be to the accuracy spec, until you get below 0.1 – 0.2 mL/L and your measured value is smaller than the nominal error margin.
While the theoretical lower limit of the instrument’s measurement range is zero, the measurement becomes less meaningful the closer your dissolved oxygen measurement gets to 2% of the calculated saturation. For example, if you were in an environment where the oxygen saturation was 7 mL/L but the oxygen concentration measured by the SBE43 was 0.28 mL/L, then your accuracy would effectively be +/- 50% of the measurement, making it relatively useless.
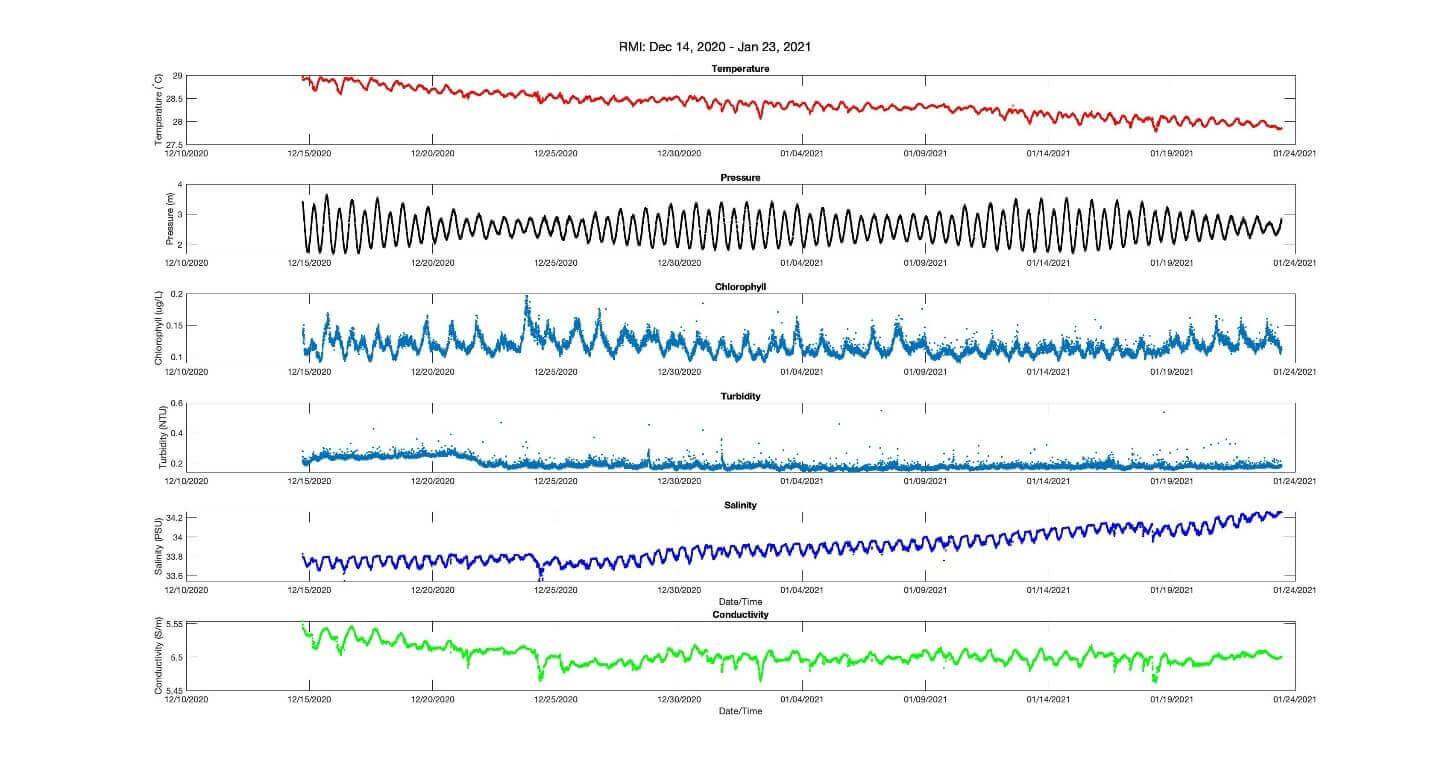
Example Data:
Temperature and Conductivity are two of the most important values taken into consideration when our instrument calculates the practical salinity of seawater. When one sees a change in a measured value, such as temperature, that change will affect your salinity reading in a predictable way, assuming all else is equal. For instance, in an environment where temperature has begun to drift downward you will see a resulting drift of salinity towards being saltier.
Proper cleaning procedures, allowing your CTD to equilibrate at the surface before a profile, updating your calibrations yearly, and bio-fouling prevention are some ways that you can ensure that your instrument will provide accurate salinity data.
A vented copper cover over the sensor can provide some protection against bio-fouling. There is a copper anti-foul guard available for standalone SeaFETs that covers the sensing surfaces.nnIf you use a SeapHOx system, the conductivity cell on the SBE 37-SMP comes equipped with TBTO anti-foul devices at the sample intake and exhaust. These devices will provide a further degree of fouling protection for the ISFET sensor in the pumped flow path. For best results, we recommend replacing the TBTO anti-foul devices before each deployment.nnThere are some antifouling systems that are not recommended:nnWe recommend against electro-chlorination systems. Stray currents in the water could damage the ISFET. Also, adding electrical potential could disrupt the feedback loop between the ISFET and the counter electrode, causing unreliable pH measurements. Finally, a electro-chlorination system doesn’t create just chlorine species, it creates any ions possible in seawater with a lower standard potential then the voltage you are applying. This will create a number of ionic species that will affect the pH of the sample, thereby causing your pH measurements to be inaccurate. In general, we would not recommend putting a ISFET pH sensor inline or downstream from a seawater electrolysis system.nnWe also recommend against UV anti-fouling systems with a SeaFET. There is a risk of exposing the chip to UV radiation, which can cause damage.
The temperature and salinity correction for the SUNA can be traced to the experiment outlined in Sakamoto et al. 2009, which is the T/S correction our UCI-based SUNA software uses in post-processing only.nnAbsorption of UV light in seawater is dominated by dissolved nitrate and bromide ions at wavelengths less than 240 nm. To estimate nitrate, it is necessary to remove the absorption due to bromide. The salinity correction addresses the sea salt extinction coefficients due to bromide. In the real ocean, bromide covaries with NaCl, so we can measure (during calibration) the the bromide absorption due to seawater at one salinity and later predict the absorption due to seawater at any salinity.nnArtifical seawater surrogates do not necessarily have the correct bromide absorption to be able to validate the the Sakamoto et al. 2009 salinity correction, so the salinity correction may not product accurate results if your data was not collected in natural seawater.
Yes, provided the SeaFETTM sensing elements remain ice-free, the instrument works across the broad temperature range of 0 to 50 deg C. The original SeaFETTM prototypes developed at MBARI and SCRIPPS were deployed extensively in the Antarctic for several studies. For example times series data, please see Matson et al. 2011.
Instrument specific calibrations are more accurate and correspond to the published accuracy specifications.
The accuracy of the class based calibration is estimated to be 2.2 uM +/- 20%.
SBE Data Processing includes a module called Seacalc III. Seacalc III can calculate density, sound velocity, and a number of other parameters for a given user input of pressure, temperature, and conductivity (or salinity).
Sea-Bird real-time data acquisition (Seasave V7) and data processing (SBE Data Processing) software supports calculation of Chen-Millero, Del Grosso, and Wilson sound velocities. The algorithms, as implemented in our software, are provided in the software documentation, which is available via the software Help files or in an Appendix in the software manuals.
The Hydrographic Society published Special Publication No. 34 in 1993, “A Comparison Between Algorithms for the Speed of Sound in Seawater”, comparing a number of sound velocity algorithms. The report recommends using the Chen-Millero algorithm for water depths less than 1000 meters and the Del Grosso algorithm for water depths greater than 1000 meters, and recommends that the Wilson algorithm should not be used. Access the report via the Hydrographic Society’s website.
Direct SV probes measure the time (flight time) required for a sound pulse to travel over a fixed length, using a high-speed clock to measure time. The clock starts when the pulse is emitted, and stops when the pulse is received. Theoretically, you only need to know the path length (and frequency of the clock ? an easy matter) to compute SV:
SV = acoustic path length / flight time.
For a typical acoustic path of 0.1 m, a flight time of 67 microseconds is expected for SV = 1492 m/s.
Two problems associated with direct SV probes are:
- The path length is not readily determined by a ruler measurement. The true length includes some depth into the acoustic transducer at which the pulse actually arises and again some depth where it is actually detected. Consider for example an typical instrument with specified accuracy of 0.05 m/s. For a typical water SV of 1500 m/s and a probe acoustic path of 100 mm, achieving this accuracy requires that length be determined to within (0.05/1500) x 100 mm = 0.003 mm (approximately 1/25 the thickness of a sheet of paper). The acoustic transducer would be of order 1 mm thick, so its dimension is much larger (300 times) than the length associated with the specified accuracy.
- Determining the actual flight time is not as simple as counting clock pulses. There are other time delays in determining both the start of the acoustic pulse and the time of its reception. Recalling that the time sound requires to travel 100 mm is approximately 67 microseconds, to measure SV to within 0.05 m, the flight time must be determined to within (0.05/1492) x 67 microseconds = 2.2 nanoseconds. It is exceedingly difficult to measure time to such precision, especially as the time lag associated with the acoustic transducer is much larger than this ? typically of order 1 microsecond (hundreds of times larger than the permitted error).
The fact is that in designing a direct path SV probe, the determination of length by ruler is only good to 5 or 10% (approximately 100 m/s equivalent uncertainty in SV). The actual determination of SV response therefore must be made in a calibration bath (using a CTD as a reference!), which is how all SV probes are calibrated.
Direct SV probes are often marketed on the principle that the measurement is based only on fundamental physical values of length and time. That is true in theory, but the practice is a different story! Direct SV probe manufacturers do not know the length (or the time) — they just fit the probe response to CTD-computed SV. There is a place for direct SV probes. Having been calibrated in water against a CTD, they do a competent job of measuring SV in other liquids. They will go on working in oil, petrol, milk, beer, etc. — liquids in which CTD measurements have no meaning.
Upon receipt of an instrument, the initial accuracy is the accuracy when comparing to a known standard. Resolution is the smallest amount of change that a sensor can see.
ITS-90 was adopted in 1990 as the temperature scale; IPTS-68 was the previous standard. The differences are related to redefining certain triple points and other melt or freeze cells that are used as the fundamental standards for temperature. Over the oceanographic ranges of temperature, a linear approximation is used to convert:
IPTS-68 = 1.00024 * ITS-90
The difference is small, but at WOCE levels it is significant.
Note: Salinity, density, and sound velocity are still defined in terms of IPTS-68 temperature. Sea-Bird’s software uses IPTS-68 temperature to calculate these derived parameters, regardless of which temperature scale you select for outputting or plotting temperature.
Application Note 42: ITS-90 Temperature Scale provides a more detailed description.
The modern oceanographic definition of salinity is the Practical Salinity Scale of 1978 (PSS-78). By definition, PSS-78 is valid only in the range of 2 to 42 psu. Sea-Bird uses the PSS-78 algorithm in our software, without regard to those limitations on the valid range.
Unesco technical papers in marine science 62 “Salinity and density of seawater: Tables for high salinities (42 to 50)” provides a method for calculating salinity in the higher range (access this paper via Unesco’s website).
The numeric difference between psu and ppt is small; both indicate ocean salinity. Prior to 1978, oceanographers referred to the physical quantity ppt (kg salt per kg water in parts per thousand). In 1978, the Practical Salinity Scale (PSS-78) was adopted, which yields a practical salinity from equations, smooth expansions of conductivity ratio, which were carefully fit to the real salinity of diluted North Atlantic seawater. The numeric unit from PSS-78 is psu (practical salinity unit). The primary motivation for psu was consistency; it focused on a trace to a primary conductivity standard (K15) and recognition that ocean ion ratios were not identical. Salinometer work was plagued by an inconsistent standard and the ppt equations included ion ratios from different oceans. So, the trade was a consistent standard and equation that works for a single ion mix instead of exact salinity in other ocean basins. G. Siedler and H. Peters highlighted where PSS-78 and EOS-80 formulas deviate from real salinity and density (e.g., Baltic Sea is difficult, but the deep Pacific has EOS-80 deviations of up to 0.02 kg/m3, implying salinity errors of order 0.02 psu).
In June 2009, a new Thermodynamic Equation of State of Seawater, referred to as TEOS-10, was adopted by the Scientific Committee on Oceanic Research and the International Association of Physical Sciences of the Ocean Working Group 127. The new equation incorporates a more accurate representation of salinity known as Absolute Salinity. Application Note 90 discusses this new equation, and Sea-Bird’s implementation in SBE Data Processing.
All data on-board the Thetis Profiler is time stamped using the on-board processor clock. This clock provides 10 ms resolution. The on-board clock is synced to the shore side host controller computer during each surface telemetry session, though the user can chose to when to update the on-board clock.
To first order, the vertical resolution is set by the vertical profiling speed. The profiling speed is user configurable (which can be changed during the deployment). At typical profiling speeds of 20 cm/sec, the Thetis Profiler provides the required 0.25 m vertical resolution (even with the slowest 1 Hz sensors).
Derivation of Kd from a
In what follows we have left off the dependence on wavelength. In addition we have left off effects due to inelastic scattering.
Modeling Kd(z) requires the inclusion of the depth dependence of the shape of the radiance
distribution. This can be accomplished by using Gershun’s equation:
K(z) = a(z) / ? ? (z)
where a(z) is the absorption coefficient and ?(z) is the average cosine of the light field.
Kd(z) differs from K(z) by only a few percent, so that we may set:
Kd(z) ? a(z) / ? ? (z) .
Berwald et al. (1995) have derived a parametric model for the dependence of ??(z) on ?o for a vertical sun in a black sky. We will assume the same depth dependence for an ordinary sky. This is not precise, but the average cosine varies slowly and has a typical range of only about 10%. The model is:
?o= b / c, where b and c are the total scattering and attenuation coefficients, including water.
? = cz, is the optical depth.
??(?) = ?? + (?(0) – ?? ) exp(-Ptt ).
?? = – 1.59 ?o4 + 1.71 ?o3 – 0.467 ?o2 – 0.347 ?o + 1
?(0)= cosine of refracted solar zenith angle
P?(?o) = – 0.166 ?o2 + 0.341 ?o + 0.0305
Berwald et. al. Limnology and Oceanography 1995, vol. 40, no8, pp. 1347-1357 .
The SeaFETTM reports pH measurements on the total scale. Values of pH reported on the total scale represent the effective hydrogen ion concentration with contributions from both free hydrogen ions and those which have reacted with sulfate.
Conductivity cells drift primarily as a function of cell fouling. There are several sources of the fouling:
- Biological growth is the primary source of cell fouling. Rinsing the conductivity cell with clean de-ionized water after each cast helps prevent most growth in the cell. If the cell is not rinsed, or standard tap water is used, growth rates can be severe. As the cell fouls, it will drift towards lower salinity values.
- Surface oil slicks also cause cell fouling. Avoid deploying the CTD through obvious slicks. When working in coastal areas, with higher chances of oil fouling, rinse and soak the cell with a 1% Triton X-100 solution (diluted in clean DI water) to help prevent oil fouling.
See Application Note 2D: Instructions for Care and Cleaning of Conductivity Cells for rinsing, cleaning, and storage procedures.
Because of the nature of fouling, the total cell drift may not be linear. It exhibits rapid small shifts (especially if related to oil fouling) on top of a base line drift. It is important to take water samples to document the behavior. Application Note 31: Computing Temperature and Conductivity Slope and Offset Correction Coefficients from Laboratory Calibrations and Salinity Bottle Samples discusses how to correct the data.
The SUNA V2 determines nitrate concentrations from the shape of the UV absorption curve. The least squares curve fitting algorithm uses calibrated extinction coefficients for nitrate and bromide (strong absorbing species in salt water) to calculate the concentration of nitrate from the UV absorption curve. The algorithm also employs a linear baseline correction that accounts for absorption that is not associated with either nitrate or bromide. The linear baseline correction successfully compensates for CDOM absorption in cases where the CDOM absorption is close to linear in the low UV. The composition of CDOM is dependent on the type of drainage area around a particular watershed and is therefore highly variable. As a result, the shape of CDOM absorption curve can vary from region to region. For this reason, the baseline correction does not always successfully compensate for CDOM absorption. In cases where the CDOM absorption curve mimics the shape of the nitrate absorption curve, a positive bias can occur.
The most common approach for correcting a positive bias caused by CDOM absorption is to correlate the continuous in situ nitrate data provided by the SUNA V2 with nitrate concentrations from discrete water quality samples measured in a laboratory. The bias may then be calculated either as an absolute offset or as a factor. In order to provide the most robust correction possible, the discrete sample size should be sufficiently large to allow for comparisons and the relationship between the in situ and discrete concentrations should be highly correlated.
The acquisition of conductivity/salinity data alongside the SeaFETTM is recommended. The acquisition of temperature data is not necessary as the SeaFETTM performs temperature measurements. Typically, conductivity and temperature measurements are combined in standard sensor packages. One can use an external temperature measurement calibrated to a relevant scale to confirm the accuracy of the SeaFETTM temperature measurement.
The proximity of the two sensors, as well as, the abruptness of spatial gradients in temperature are both important considerations.
Sea-Bird Scientific multispectral 500 series radiometers measure light at each fixed wavelength with an interference filter/detector assembly. The analog output of each detector is amplified and digitized. The amplification stage and noise filtering is fine tuned for each wavelength to produce an optimal saturation limit and frame rate. This maximizes the signal to noise ratio while ensuring that each channel does not saturate during normal operations. The frame rate of each radiometer is fixed anywhere between 1 and 24 Hz depending on the customers specific requirements. 4 and 7 channel radiometers can be purchased in several configurations with different field of views. They have a small diameter to reduce self-shading and generate a digital output for stand-alone operations or they can operate as part of a larger 485 network of sensors (SATNet). 500 series sensors are also very low power devices making them excellent sensors for power limited platforms such as buoys, AUV’s and profiler floats.
Sea-Bird Scientific Hyperspectral HOCR radiometers use a Zeiss spectrograph optimally configured and characterized to measure light between 350 and 800 nm (approximately 136 individual channels). With the HOCR series, a variable integration time is used for all channels in the array and upper and lower thresholds are set so that no channel saturates within that array. Thermal dark current changes that occur within the spectrograph are corrected across the full spectrum with the use of a mechanical dark shutter that closes periodically in the radiometer. A separate frame of data is generated for this dark reading. Frame rates are dependent on the integration time of the device so are considered variable. When light levels are high, the integration time and frame rate are also high, so that you are collecting many frames per second. As the light level decreases, the integration time must increase and therefore the frame rate becomes longer. Integration times range from 4 ms to 2 seconds. HOCR sensors also have a small diameter to reduce self-shading and the same telemetry options are offered. Sea-Bird Scientific also offers a low power, non-SATNet version of the HOCR sensor for remote platforms that are power limited.
Sea-Bird Scientific has developed cosine collectors that are specifically designed to optimize performance in the intended media of operation (air or water). So in water irradiance sensors have cosine collectors that provide an excellent response in water but not in air. In air irradiance sensors provide an excellent cosine response in air but not in water.
The FIRe System measures changes in chlorophyll fluorescence that occur during a short (100 – 400 ?s) but intense (> 20,000 ?mol photons m-2 s-1) flash of light whereas the PAM approach measures the fluorescence induced by a weak modulated light source while using ‘saturating’ pulses of ~3000 – 10,000 ?mol photons m-2 s-1 to modify fluorescence yields.
The FIRe System also fundamentally differs from a PAM in that the FIRe fully reduces the primary electron acceptor, QA, allowing a simultaneous single closure (STF) event of all photosystem II (PSII) reaction centers whereas the PAM technique generates multiple photochemical charge separations (MTF) that fully reduces QA, the secondary acceptor, QB, and plastoquinone (PQ). By lengthening the measuring protocol the FIRe can also yield MTF data.
For a complete discussion on the mechanistic and practical differences between the two techniques see: Suggett, D.J., K. Oxborough, N.R. Baker, H.L. MacIntyre, T.M. Kana, & R.J. Geider. 2003. Fast repetition rate and pulse amplitude modulation chlorophyll a fluorescence measurements for assessment of photosynthesis electron transport in marine phytoplankton. European Journal of Phycology. 38: 371-84.
One of the reasons that this is not a simple question is that there are several factors to take into consideration regarding the error margin for practical salinity measurements. Salinity itself is a derived measurement from temperature, conductivity, and pressure, so any errors in these sensors can propagate to salinity. For example, our initial accuracy specification for the SBE 3plus temperature sensor and SBE 4 conductivity sensor on an SBE 9plus CTD is approximately equivalent to an initial salinity accuracy of 0.003 PSU (note that conductivity units of mS/cm are roughly equivalent in terms of magnitude to PSU).
However, another issue to consider is that this accuracy is defined for a clean, well-mixed calibration bath. In the ocean, some of the biggest factors that impact salinity accuracy are 1) sensor drift from biofouling or surface oils for conductivity in particular and 2) dynamic errors that can occur on moving platforms, particularly when conditions are rapidly changing, which will be true for all sensors that measure salinity. Sea-Bird provides recommendations, design features such as a pumped flow path, and data processing routines to align and improve data for the salinity calculation to account for thermal transients and hysteresis, and to match sensor response times. Depending on the environment and the steepness of the gradient, and after careful data processing, this may continue to have an impact on salinity on the order of 0.002 PSU or more, for example. For more details, see Application Note 82.
Lastly, note that salinity in PSU is calculated according to the Practical Salinity Scale (PSS-78), which is defined as valid for salinity ranges from 2 – 42 PSU.
The difference between downcast and upcast is most likely related to package wake. When the CTD is mounted under a large water sampler, the variation can be on the order of 5 to 8 meters. This is due to the shadowing of the CTD sensors by the water sampler.
For SBE 4 conductivity calibrations, Sea-Bird uses natural seawater that has been carefully collected, stored, UV irradiated, and filtered. Artificial seawater is not adequate if calibration errors are to be kept below 0.010 psu.
Note: SBE 4 is the conductivity sensor in the SBE 9plus, 25, and 25plus profiling CTDs.
The primary difference between natural and artificial seawater is the behavior of conductivity versus temperature. The practical salinity scale 1978 equations include a term rt. This term is expanded into a fourth order equation that describes the variation of conductivity versus temperature for a sample of constant salinity. The equation’s coefficients are derived by fitting to natural seawater samples. Artificial seawater does not have the same conductivity versus temperature characteristic, providing incorrect coefficients and causing a slope error in the calibration.
For calibrations of conductivity sensors other than the SBE 4, Sea-Bird uses artificial seawater (NaCl solution). However, we place an SBE 4 conductivity sensor in each bath, providing a standard for reference to the natural seawater calibration. This allows us to correct errors in the coefficients and slope introduced with the artificial seawater calibration.
For calibration of temperature sensors, Sea-Bird uses artificial seawater (NaCl solution).
The SUNA V2 contains a 256 channel spectrometer that is programmed to integrate for a specific length of time (usually 300 – 500 ms) while sampling to maximize signal. That is, when the SUNA V2 takes a sample, the spectrograph collects UV light for the length of the integration period. In optically dense waters (e.g. high turbidity or CDOM), very little UV light is transmitted through the water and therefore the spectrometer “sees” a much lower signal. The new SUNA V2 is programmed to automatically increase the integration period to compensate for the low light levels. This enables the instrument to collect a strong signal in extreme environmental conditions.
First off, let’s see what a relatively good Raw Data Plot looks like.

The different phases are the pre-flush phase (dark blue), the ambient baseline reading period (red), the sample reagent pump phase (green), the sample read/reaction phase (purple), and finally the post-flush phase (dark blue). During a “spiked run” the full set of phases are repeated twice.
Using this as an example, you want to see a flat pre- and post-pump phase, a stable (flat) ambient read phase, and a pronounced smooth reaction curve. You may see small bumps here and there due to very small bubbles or other effects but this can be negligible.
The most common failure mode is the bubble spike flag, which triggers when air is introduced into the system, most commonly through the sample line but possibly through incorrectly installed cartridges or tubing.

The bubble failure is typically identified by large jumps and spikes randomly in the data, primarily in the pre- and post- pump phase and the ambient read phase. Air bubbles create an unstable base line (red) which will affect the sensor’s accuracy. In extreme examples it can completely overshadow the reaction curve as well or shift the baseline to the point where your reaction creates a negative value. Air bubbles often exacerbate other issues, like weak pumps, clogged filters, or already low phosphate in your sample.
Since bubbles can come from a few different places there are multiple checks you can do to try to stop bubbles from appearing in your data. The most important check is ensuring that your hydrocycle is fully submerged for the entirety of your deployment, as the sample line being exposed to air will introduce bubbles.
You should check that each of your reagent carts are fully installed. Press down on each reagent cart until you can hear them click and be careful about applying too much force to any of the plastic pieces that may crack and allow for leaks or air.
You can also test the pump volumes of each reagent and sample to see if your filters are clogged or if there is another issue causing the sample injection system to fail, like a leak. You do so by using Cyclehost’s Pump controls. You should choose all pumps and run each for 100 pumps. A healthy system will pump 1-1.3 ml of reagent and 2.5-3 ml of sample.
If you have bubbles already and need to recover, the most thorough method is to follow our maintenance and cleaning instructions in the manual, primarily the extended flush. Running the extended flush multiple times should be enough to clear bubbles from your system if no other issues are evident.
If doing so still fails to recover your instrument’s counts please reach out to technical support with a copy of your hydrocycle data and brief timeline of events.
Manufacturing
The pH sensor will be shipped dry but was pre-conditioned in seawater (generally from Pacific Ocean waters near Hawaii). While conditioning and evaluating the pH sensor, only expose it filtered, sterilized natural seawater. Do not use seawater CRMs (Certified Reference Material), synthetic seawater, deionized water, NaCl Solutions, or tap water.
Before pre-deployment testing, you will need to fill the plumbing around the pH sensor with natural seawater. The pH sensor needs time to acclimate to the ionic concentration of region specific waters. Once wet, the time to recondition the sensor so that it will report within its accuracy specification depends on several factors, including the ionic composition of the seawater used and the amount of time the pH sensor was stored dry. This time can range from several hours to up to three days.
When the seawater bridge between Counter Electrode and ISFET is broken for longer than 10 seconds, it will be necessary to re-condition the sensor. The sensor does not require recalibration after being re-conditioned.
To prepare the sensor for deployment, it is recommended that several days prior to deployment, the isolated battery is connected via the float interface and the pH sensor is stored in water that is similar to the deployment site. The sensor should be stored dry to avoid bio-fouling of the ISFET and the battery may be removed during storage. Seawater creates a half cell bridge between the Counter Electrode and ISFET, and power to that circuit is provided by the isolated 9V cell. Without seawater, the battery is unnecessary and may be disconnected.
Ordering
The pH sensor will be shipped dry but was pre-conditioned in seawater (generally from Pacific Ocean waters near Hawaii). While conditioning and evaluating the pH sensor, only expose it filtered, sterilized natural seawater. Do not use seawater CRMs (Certified Reference Material), synthetic seawater, deionized water, NaCl Solutions, or tap water.
Before pre-deployment testing, you will need to fill the plumbing around the pH sensor with natural seawater. The pH sensor needs time to acclimate to the ionic concentration of region specific waters. Once wet, the time to recondition the sensor so that it will report within its accuracy specification depends on several factors, including the ionic composition of the seawater used and the amount of time the pH sensor was stored dry. This time can range from several hours to up to three days.
When the seawater bridge between Counter Electrode and ISFET is broken for longer than 10 seconds, it will be necessary to re-condition the sensor. The sensor does not require recalibration after being re-conditioned.
To prepare the sensor for deployment, it is recommended that several days prior to deployment, the isolated battery is connected via the float interface and the pH sensor is stored in water that is similar to the deployment site. The sensor should be stored dry to avoid bio-fouling of the ISFET and the battery may be removed during storage. Seawater creates a half cell bridge between the Counter Electrode and ISFET, and power to that circuit is provided by the isolated 9V cell. Without seawater, the battery is unnecessary and may be disconnected.
The SeaFET and SeapHOx systems are designed to sample at a fixed depth. If you want to run discreet samples at depth intervals, you will need to find a way to move the system to a specific depth before each sample interval and stop the descent / ascent for the entire pumping and sampling cycle to get a valid CTD / pH / Oxygen sample.
If you are able to communicate with the system through the serial I/O during profiling, you can send a sampling command to the sensor at each depth point and allow it to complete its sample cycle. Consult the manual for each model for the length of time required to complete each sample. Once the sensor provides a sample, you can then move it to the next depth point and repeat.
If you aren’t running real-time communications to the SeaFET/SeapHOx, you could also set it to autonomously sample at a time interval that gives you enough time to move the package to a new depth point between sample cycles. The challenges with this approach would be to know exactly when the sensor is sampling without any direct feedback from the instrument.
SUNAs ordered with the 5mm path length coupler as a factory option will perform much better in low light transmission waters due to the shorter length the light needs to travel leading to less absorption. Equipping your SUNA with the factory bio-wiper option will also perform better and be less susceptible biofouling or buildup of other material that can reduce light transmission.
There are also some maintenance practices and device settings that can give SUNA a better probability of being able to capture enough light for a sample. Enable adaptive integration will trigger the SUNA to increase the lamp on time when light received by the spectrometer is low. It is also important to clean the windows as frequently as possible and monitor lens for scratches. Finally, you want your maximum light spectral counts at the peak wavelength (around 240nm) to be between 45,000 and 55,000 counts in pure or deionized water. This can be viewed in the “Spectra” tab in UCI when sampling or replaying data. If your peak spectrometer output is below 45,000 counts after cleaning the window, you may increase the integration period by 25 to 50 ms if needed (but not more; further changes require a factory recalibration). After adjusting the integration period, always perform a reference spectrum update per the instructions in the SUNA manual.
Service
The pH sensor will be shipped dry but was pre-conditioned in seawater (generally from Pacific Ocean waters near Hawaii). While conditioning and evaluating the pH sensor, only expose it filtered, sterilized natural seawater. Do not use seawater CRMs (Certified Reference Material), synthetic seawater, deionized water, NaCl Solutions, or tap water.
Before pre-deployment testing, you will need to fill the plumbing around the pH sensor with natural seawater. The pH sensor needs time to acclimate to the ionic concentration of region specific waters. Once wet, the time to recondition the sensor so that it will report within its accuracy specification depends on several factors, including the ionic composition of the seawater used and the amount of time the pH sensor was stored dry. This time can range from several hours to up to three days.
When the seawater bridge between Counter Electrode and ISFET is broken for longer than 10 seconds, it will be necessary to re-condition the sensor. The sensor does not require recalibration after being re-conditioned.
To prepare the sensor for deployment, it is recommended that several days prior to deployment, the isolated battery is connected via the float interface and the pH sensor is stored in water that is similar to the deployment site. The sensor should be stored dry to avoid bio-fouling of the ISFET and the battery may be removed during storage. Seawater creates a half cell bridge between the Counter Electrode and ISFET, and power to that circuit is provided by the isolated 9V cell. Without seawater, the battery is unnecessary and may be disconnected.
Cells that have been contaminated with foreign material generally read low of the actual conductivity. Your zero (in air) conductivity reading is generally unaffected.
The conductivity error due to fouling will generally be proportional to the conductivity value. Conductivity is corrected not as an offset but as a ratio (multiplicative) error compared to a reference.
Salinity is a derivative measurement of temperature, conductivity, and pressure, and should be corrected by adjusting the component measurements. Generally speaking, an error in the conductivity measurement will correlate to a directly proportional error in the salinity measurement.
SUNAs ordered with the 5mm path length coupler as a factory option will perform much better in low light transmission waters due to the shorter length the light needs to travel leading to less absorption. Equipping your SUNA with the factory bio-wiper option will also perform better and be less susceptible biofouling or buildup of other material that can reduce light transmission.
There are also some maintenance practices and device settings that can give SUNA a better probability of being able to capture enough light for a sample. Enable adaptive integration will trigger the SUNA to increase the lamp on time when light received by the spectrometer is low. It is also important to clean the windows as frequently as possible and monitor lens for scratches. Finally, you want your maximum light spectral counts at the peak wavelength (around 240nm) to be between 45,000 and 55,000 counts in pure or deionized water. This can be viewed in the “Spectra” tab in UCI when sampling or replaying data. If your peak spectrometer output is below 45,000 counts after cleaning the window, you may increase the integration period by 25 to 50 ms if needed (but not more; further changes require a factory recalibration). After adjusting the integration period, always perform a reference spectrum update per the instructions in the SUNA manual.
Field Procedures & Deployment
The pH sensor will be shipped dry but was pre-conditioned in seawater (generally from Pacific Ocean waters near Hawaii). While conditioning and evaluating the pH sensor, only expose it filtered, sterilized natural seawater. Do not use seawater CRMs (Certified Reference Material), synthetic seawater, deionized water, NaCl Solutions, or tap water.
Before pre-deployment testing, you will need to fill the plumbing around the pH sensor with natural seawater. The pH sensor needs time to acclimate to the ionic concentration of region specific waters. Once wet, the time to recondition the sensor so that it will report within its accuracy specification depends on several factors, including the ionic composition of the seawater used and the amount of time the pH sensor was stored dry. This time can range from several hours to up to three days.
When the seawater bridge between Counter Electrode and ISFET is broken for longer than 10 seconds, it will be necessary to re-condition the sensor. The sensor does not require recalibration after being re-conditioned.
To prepare the sensor for deployment, it is recommended that several days prior to deployment, the isolated battery is connected via the float interface and the pH sensor is stored in water that is similar to the deployment site. The sensor should be stored dry to avoid bio-fouling of the ISFET and the battery may be removed during storage. Seawater creates a half cell bridge between the Counter Electrode and ISFET, and power to that circuit is provided by the isolated 9V cell. Without seawater, the battery is unnecessary and may be disconnected.
Cells that have been contaminated with foreign material generally read low of the actual conductivity. Your zero (in air) conductivity reading is generally unaffected.
The conductivity error due to fouling will generally be proportional to the conductivity value. Conductivity is corrected not as an offset but as a ratio (multiplicative) error compared to a reference.
Salinity is a derivative measurement of temperature, conductivity, and pressure, and should be corrected by adjusting the component measurements. Generally speaking, an error in the conductivity measurement will correlate to a directly proportional error in the salinity measurement.
The SeaFET and SeapHOx systems are designed to sample at a fixed depth. If you want to run discreet samples at depth intervals, you will need to find a way to move the system to a specific depth before each sample interval and stop the descent / ascent for the entire pumping and sampling cycle to get a valid CTD / pH / Oxygen sample.
If you are able to communicate with the system through the serial I/O during profiling, you can send a sampling command to the sensor at each depth point and allow it to complete its sample cycle. Consult the manual for each model for the length of time required to complete each sample. Once the sensor provides a sample, you can then move it to the next depth point and repeat.
If you aren’t running real-time communications to the SeaFET/SeapHOx, you could also set it to autonomously sample at a time interval that gives you enough time to move the package to a new depth point between sample cycles. The challenges with this approach would be to know exactly when the sensor is sampling without any direct feedback from the instrument.
SUNAs ordered with the 5mm path length coupler as a factory option will perform much better in low light transmission waters due to the shorter length the light needs to travel leading to less absorption. Equipping your SUNA with the factory bio-wiper option will also perform better and be less susceptible biofouling or buildup of other material that can reduce light transmission.
There are also some maintenance practices and device settings that can give SUNA a better probability of being able to capture enough light for a sample. Enable adaptive integration will trigger the SUNA to increase the lamp on time when light received by the spectrometer is low. It is also important to clean the windows as frequently as possible and monitor lens for scratches. Finally, you want your maximum light spectral counts at the peak wavelength (around 240nm) to be between 45,000 and 55,000 counts in pure or deionized water. This can be viewed in the “Spectra” tab in UCI when sampling or replaying data. If your peak spectrometer output is below 45,000 counts after cleaning the window, you may increase the integration period by 25 to 50 ms if needed (but not more; further changes require a factory recalibration). After adjusting the integration period, always perform a reference spectrum update per the instructions in the SUNA manual.
The ECO-PAR, due to the nature of PAR sensors, cannot be accurately calibrated outside of the Sea-Bird facility. However, there are some functionality tests that can aid in pre-deployment. A bright flashlight can validate whether the instrument sees light at all.
On a bench test one should see between 1000-4000 counts normally with the instrument in the white cap standing up on the benchtop. Use a terminal program to see the raw counts, such as Tera Term, and point a flashlight beam near the white cap. Doing so with a functioning unit will cause the counts to go to approximately a couple of thousand in a hair trigger fashion. It should be possible to decrease the counts on a properly functioning instrument by cupping ones hands around the white cap to shield it from light. It should be easy to get a response of a couple of hundred counts total in doing this. In the field if you shine a flashlight beam directly into the optics will see low level ambient light and it is easy to regulate the output in counts at the low end of the range (less than 1000 counts) when it is functioning normally. If your unit is not properly functioning, it will go from 50-ish to a couple of thousand counts and it will be very difficult or impossible to get an output of a couple of hundred counts. While the ranges of the response may differ between PAR models, these tests can be used with other PAR sensors to verify operation.
There are two optional modifications that can be done to your 9p at our facility (during service or as part of your original order) that will allow the 9p CTD to operate in freshwater deployments. The 9p’s pump will not operate in freshwater without these options.
First, the Modem Pump Control, allows you to control the pump directly, bypassing the requirement for it to see a certain conductivity frequency to activate. (On other CTD’s we can change this conductivity value to allow both freshwater and saltwater).
The second option is the freshwater contact pin, an optional pin modification that allows for the detection of fresh water by the 9p.
First off, let’s see what a relatively good Raw Data Plot looks like.

The different phases are the pre-flush phase (dark blue), the ambient baseline reading period (red), the sample reagent pump phase (green), the sample read/reaction phase (purple), and finally the post-flush phase (dark blue). During a “spiked run” the full set of phases are repeated twice.
Using this as an example, you want to see a flat pre- and post-pump phase, a stable (flat) ambient read phase, and a pronounced smooth reaction curve. You may see small bumps here and there due to very small bubbles or other effects but this can be negligible.
The most common failure mode is the bubble spike flag, which triggers when air is introduced into the system, most commonly through the sample line but possibly through incorrectly installed cartridges or tubing.

The bubble failure is typically identified by large jumps and spikes randomly in the data, primarily in the pre- and post- pump phase and the ambient read phase. Air bubbles create an unstable base line (red) which will affect the sensor’s accuracy. In extreme examples it can completely overshadow the reaction curve as well or shift the baseline to the point where your reaction creates a negative value. Air bubbles often exacerbate other issues, like weak pumps, clogged filters, or already low phosphate in your sample.
Since bubbles can come from a few different places there are multiple checks you can do to try to stop bubbles from appearing in your data. The most important check is ensuring that your hydrocycle is fully submerged for the entirety of your deployment, as the sample line being exposed to air will introduce bubbles.
You should check that each of your reagent carts are fully installed. Press down on each reagent cart until you can hear them click and be careful about applying too much force to any of the plastic pieces that may crack and allow for leaks or air.
You can also test the pump volumes of each reagent and sample to see if your filters are clogged or if there is another issue causing the sample injection system to fail, like a leak. You do so by using Cyclehost’s Pump controls. You should choose all pumps and run each for 100 pumps. A healthy system will pump 1-1.3 ml of reagent and 2.5-3 ml of sample.
If you have bubbles already and need to recover, the most thorough method is to follow our maintenance and cleaning instructions in the manual, primarily the extended flush. Running the extended flush multiple times should be enough to clear bubbles from your system if no other issues are evident.
If doing so still fails to recover your instrument’s counts please reach out to technical support with a copy of your hydrocycle data and brief timeline of events.
Recent FAQs
Scientific
The ECO sensors primarily image a volume that is approximately 1 cm3, centered 1 cm off the face of the instrument.
NOTE: This does not preclude return of photons from outside of this volume and in particular it is best that no fixed objects are in the field of view of the instrument, such as cables or cage hardware.
We recommend that the instruments be mounted in such a way that they are seeing only the free field. Often when mounted on a CTD, mounting the ECO face-down or away from the CTD can improve reading accuracy.
That said, the ECO’s have been used in fairly tight quarters with excellent results, including in flow-through housings and dense instrument cages.
When the ECO is mounted in such a way that there is an object in front of the Instrument, the ‘wall effect’ should be established. We are assuming that the material does not fluoresce.
To establish the “wall effect”:
1) Turn the instrument on in air, or better, in a clear water bath, and collect an ‘offset’ reading.
2) Compare this to the factory calibration offset. If the difference is small (e.g. a few counts or mV), then no further action is necessary.
3) Rotate the instrument to find the minimum offset. The backscattering or turbidity channel is the best for this.
4) Mark this position and record the output values of all channels.
5) To minimize the wall effect any object in front of the face of the instrument should be dull black or grey. Tape is usually the easiest solution for this on frames. Grey or black matte plastic is the solution for underway systems.
6) Use these values as the offset values in generating engineering unit output, as specified on the characterization sheets:
Output = ScaleFactor x [InstrumentOutput – Offset]
7) After collecting data from the field, check to confirm that the offset you are using is appropriate. You may find that your minimum values are lower than the offset you have established.
The deep SBE41 and SBE61 use the same pressure sensor – a 7000dbar Kistler. And, they are calibrated with the same Paroscientific Digiquartz reference. However, the calibration process is different. A deep SBE41 receives a 2-point sensor only temperature compensation for pressure. The initial accuracy for a deep 41 is +/- 7dbar, typical stability is 2dbar/year. A 61 receives a 4-point temperature compensation for pressure after the instrument is completely assembled, such that the correction includes both the sensor and the electronic boards. The initial accuracy for a 61 is +/-4.5dbar, typical stability is 0.8dbar/year.
The pH sensor will be shipped dry but was pre-conditioned in seawater (generally from Pacific Ocean waters near Hawaii). While conditioning and evaluating the pH sensor, only expose it filtered, sterilized natural seawater. Do not use seawater CRMs (Certified Reference Material), synthetic seawater, deionized water, NaCl Solutions, or tap water.
Before pre-deployment testing, you will need to fill the plumbing around the pH sensor with natural seawater. The pH sensor needs time to acclimate to the ionic concentration of region specific waters. Once wet, the time to recondition the sensor so that it will report within its accuracy specification depends on several factors, including the ionic composition of the seawater used and the amount of time the pH sensor was stored dry. This time can range from several hours to up to three days.
When the seawater bridge between Counter Electrode and ISFET is broken for longer than 10 seconds, it will be necessary to re-condition the sensor. The sensor does not require recalibration after being re-conditioned.
To prepare the sensor for deployment, it is recommended that several days prior to deployment, the isolated battery is connected via the float interface and the pH sensor is stored in water that is similar to the deployment site. The sensor should be stored dry to avoid bio-fouling of the ISFET and the battery may be removed during storage. Seawater creates a half cell bridge between the Counter Electrode and ISFET, and power to that circuit is provided by the isolated 9V cell. Without seawater, the battery is unnecessary and may be disconnected.